Explain the Difference Between a Cation and an Anion
Cation and anion attract each other to form an ionic bond. Sometimes the cation or anion is a polyatomic ion.

What Is The Difference Between Cation Exchange Capacity And Anion Exchange Capacity Compare The Difference Between Similar Terms
An amino acid has this ability because at a certain pH value different for each amino acid nearly all the amino acid molecules exist as zwitterions.
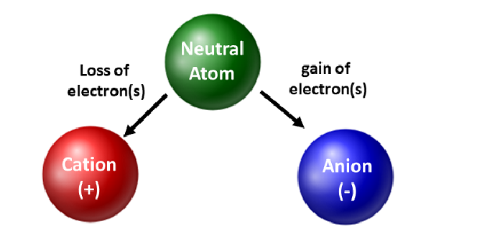
. As you travel across the PT the atomic number increases because the number of p are increasing with each consecutive atom. There are only 3 cation polyatomic ions and they are ammonium NH 4 hydronium H 3 and mercuryI Hg 2 2. This is depicted in Figure 2 as shown below where the.
The individual molecules can have. So we can say that ionic bonds are an electrostatic force of attraction between oppositely charged ions. Covalent bonds are formed by equal sharing of electrons between.
EqMgCl_2 eq Magnesium Chloride. The magnesium is a metal cation and the chlorine is a non-metal anion. Types of Chemical Bonds Ionic bonding.
Explain the difference in first ionization energy between lithium and beryllium. The structure of an amino acid allows it to act as both an acid and a base. Although there are differences between laboratories and assays the normal anion gap has traditionally been set between 8 mEqL to 12 mEqL.
The SLC22 subfamily also includes the organic cation transporters OCTs and organic carnitine zwitterion transporters OCTNs. Compounds that contain carbon also called organic compounds. Non-covalent electrostatic interactions can be strong and act at long range.
An ionic bond can also be between two molecules. The nucleus of the Be atom has a greater positive charge thus wants to hold on more tightly to the 2 e-. But when a single cation and a single anion are close together within a protein or within a folded RNA those interactions are considered to be non-covalent electrostatic interactions.
Oppe x i SnFq u- 19. One of the atoms is a cation which is smaller in size and the other atom is an anion which is a lot larger in size. The greater the charge disparity between the cation and the anion the stronger the ionic bond.
OAT family members are highly similar. The ionic bond gains strength from the difference in charge between the two atoms ie. Electrostatic forces fall off gradually with distance 1r 2 where r is the distance between the ions.
Theres no good trick to remembering these you just need to memorize them. Coordination isomerism occurs in compounds containing complex anionic and complex cationic parts and can be viewed as an interchange of some ligands from the cation to the anion. NaCl is an ionic compound in which Na and Cl combine to form an ionic compound.
They all have a 1 charge though technically 2. These are molecules that have two or more atoms with ionic groups. Explain how an amino acid can act as both an acid and a base.
The capsule and tubule are connected and are composed of. So in order to account for this difference one most get the total distance between the two nuclei and divide the distance according to atomic size. Figure 3 shows.
The organic anion transporter OAT family comprises a group of over 10 transmembrane proteins Table 1 falling into the SLC22 solute carrier 22 subfamily of the major facilitator superfamily MFS. If the anion gap is greater than 12 this suggests an increased presence of unmeasured anions. The bigger the atomic size the larger radius it will have.
Hence there are two complex compounds bound together one with a negative charge and the other with a positive charge. In coordination isomers the anion and cation complexes. The size of an anion is larger than that of the parent atom as the addition of one or more electrons would result in increased repulsion among the electrons and a decrease in effective nuclear charge.
Determination of the source of said anions while somewhat dependent on a high-quality history and physical exam generally. Support your answer by citing two specific examples. Do the Roman numerals in the names in Model 3 relate to the number of cations or number of anions In the formula unit.
Keeping in mind that the sum of the charges in an. A cation is smaller than the parent atom as it possesses fewer electrons whereas its nuclear charge remains the same. The nephron is the minute or microscopic structural and functional unit of the kidneyIt is composed of a renal corpuscle and a renal tubuleThe renal corpuscle consists of a tuft of capillaries called a glomerulus and a cup-shaped structure called Bowmans capsuleThe renal tubule extends from the capsule.
Describe the most obvious difference between the names in Model 3 and those in Model 2. With the increase in p comes an increase in nuclear charge. The excess capacity equals the difference between initial charge capacity and capacity from conventional transition metal redox that is Ni 2 to Ni 3 Ni 3 to Ni 4 and Co 3 to Co 4.
A covalent bond indicates the sharing of electrons between atoms. If acid is added to a solution containing the zwitterion the carboxylate group.

10 Differences Between Cations And Anions With Examples Viva Differences

Difference Between A Cation And An Anion School Anion Cation Difference School Hausdekorati Chemistry Classroom Chemistry Basics Chemistry Education
Difference Between Cation And Anion Psiberg

Difference Between Anion And Cation Compare The Difference Between Similar Terms

Cation Vs Anion What S The Difference Dictionary Com

Ions Cation Anion Aboodytv Chemistry Youtube

Difference Between Cation And Anion In Tabular Form
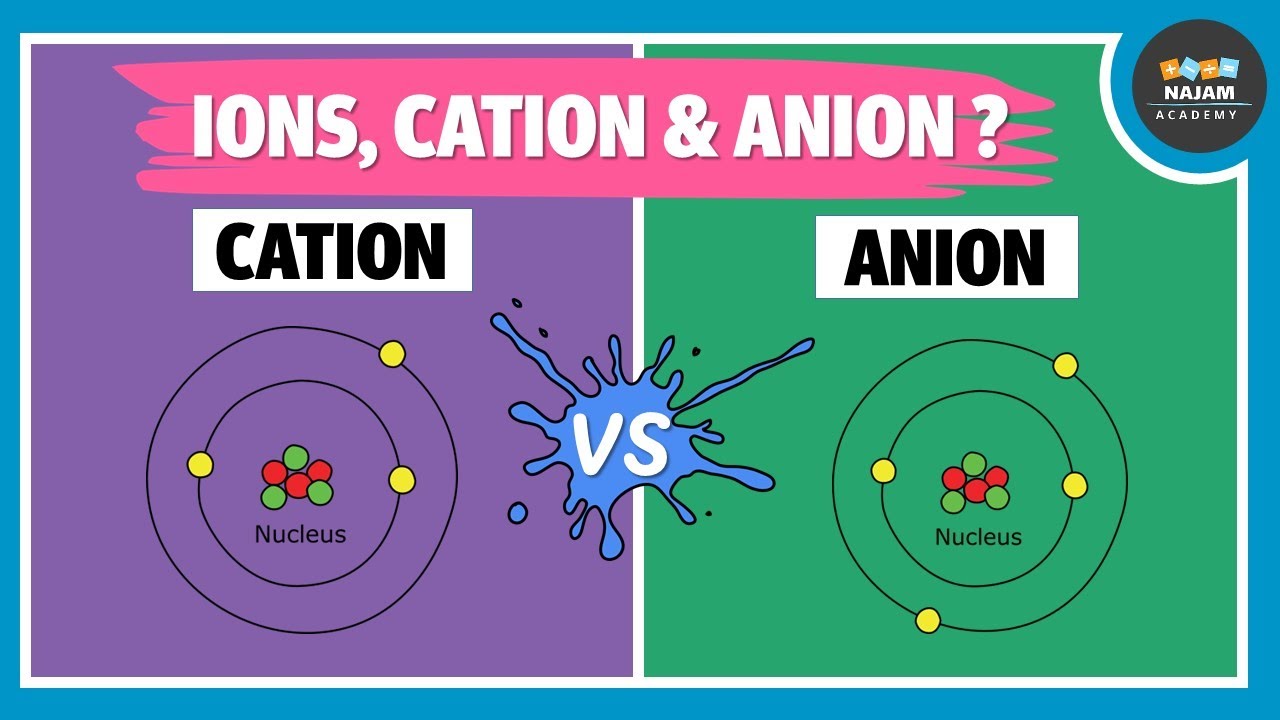
What Is An Ion Cation And Anion Chemistry Youtube
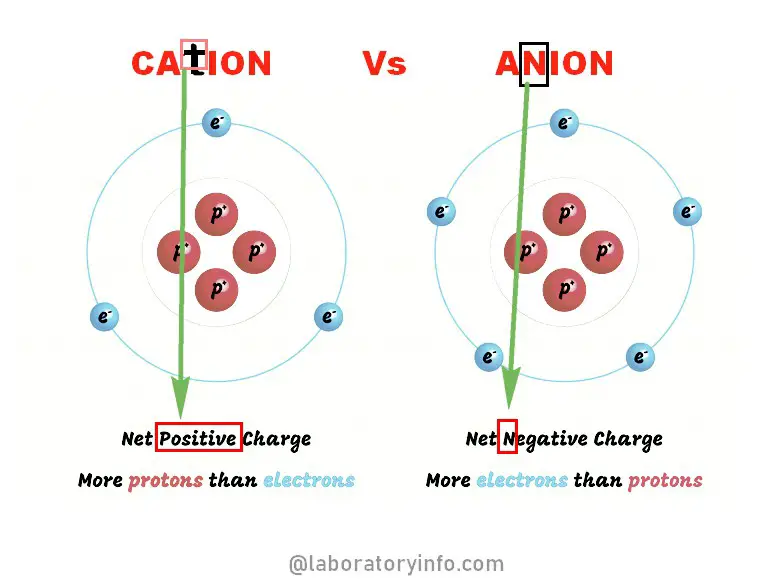
Difference Between Cation And Anion Laboratoryinfo Com

Do You Know How To Tell Cation And Anion Ions Apart Chemistry Help Chemistry Education Chemistry Lessons

What Is The Difference Between A Cation And Anion Snapsolve

What Is The Difference Between Cation And Anion In Urdu Hindi Types Of Ions Youtube
Difference Between Cation And Anion
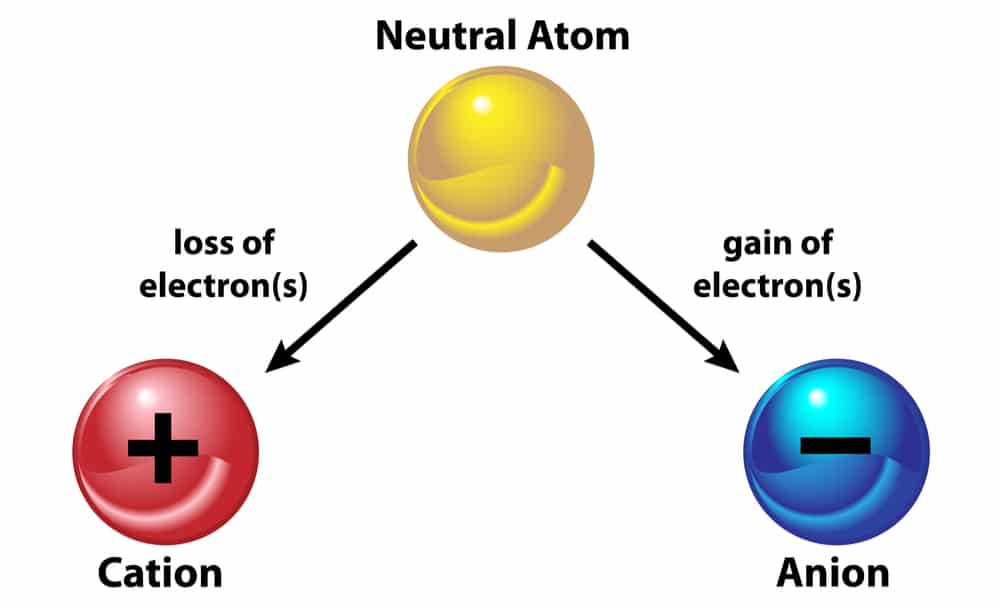
The Anion Cation Connection In Soil Ecofarming Daily
Difference Between Cation And Anion
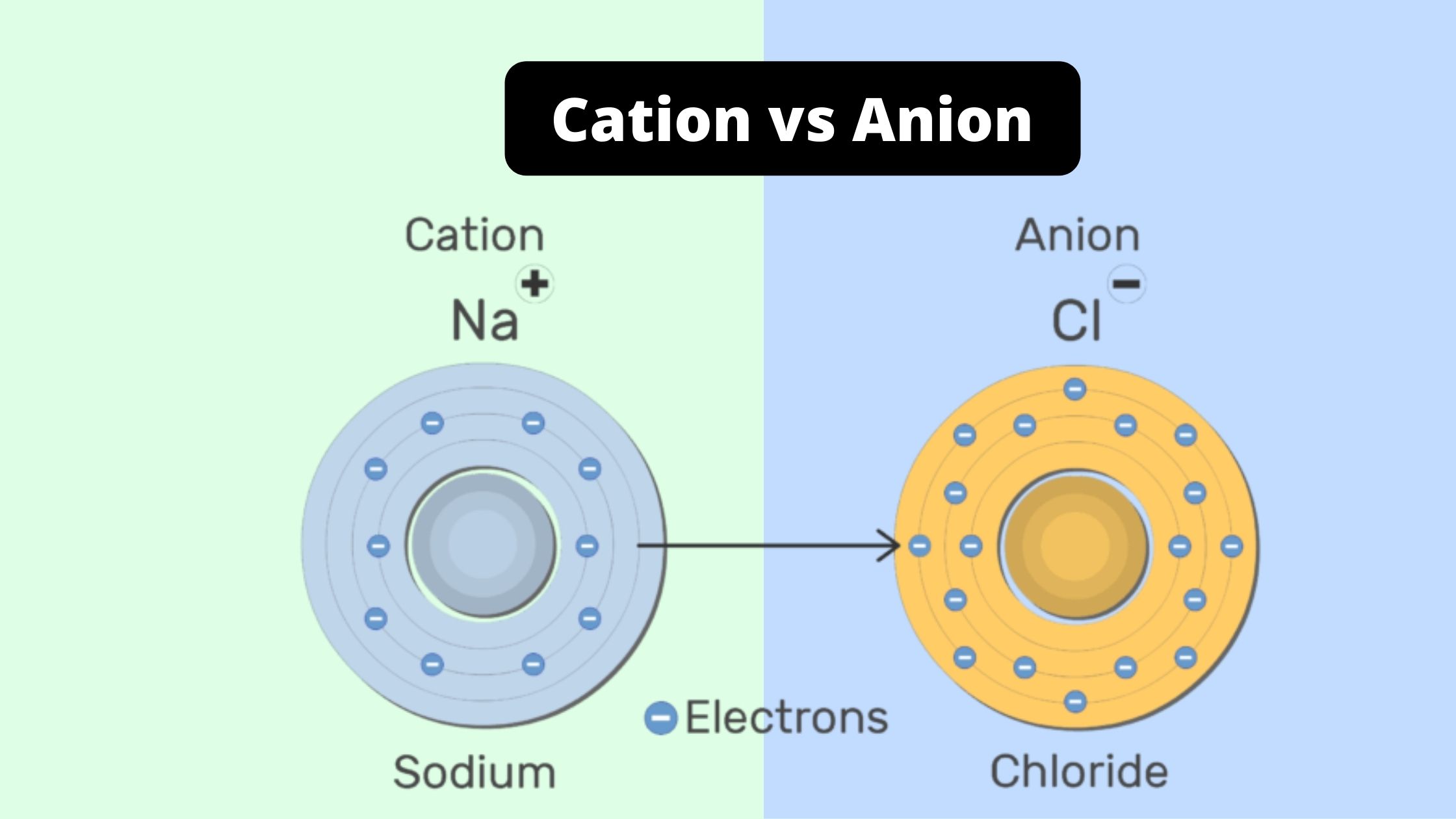
Difference Between Cation And Anion Cation Vs Anion
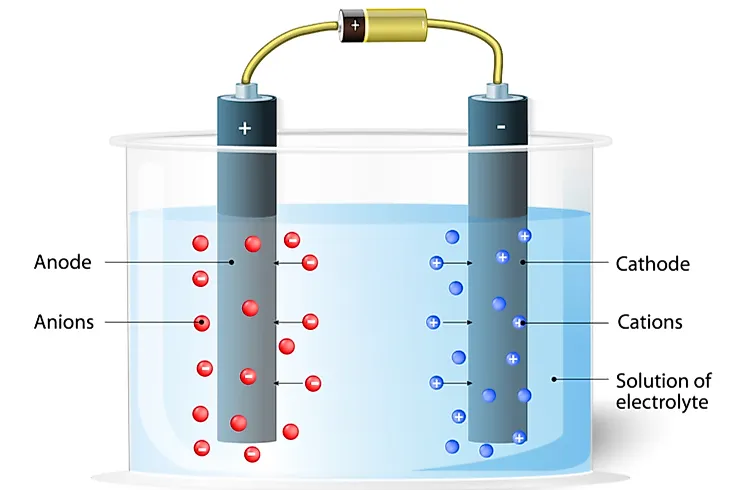
What Is The Difference Between A Cation And An Anion Worldatlas

Cations And Anions Difference Between Anions And Cations With Examples
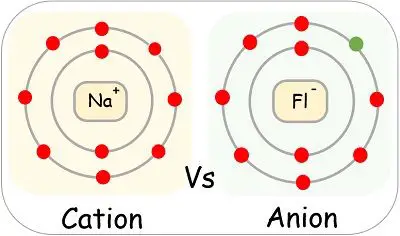
Comments
Post a Comment